Total-Internal-Reflection-Fluorescence Microscopy with Quartz-Crystal Microbalance
Examining the reactions between proteins is very important in drug discovery. For example, when a protein causing a disease (target) is identified, a protein with a strong affinity for the protein will be examined as a candidate for use as an agent. That's why knowing which protein bonds to which part of the target and with what bonding force is very important in assessing the ability of the protein as an agent.
Researchers have tried to integrate a system for measuring affinity between proteins with a fluorescent microscope, but they were unable to achieve this due to a metal coating on the chip prevented optical penetration.
We study aggregation phenomenon of Aβ peptides highly associated with pathogenesis of Alzheimer's disease (AD). In order to clarify the fibrillation mechanism of amyloid β (Aβ) peptides, this group has succeeded in developing a novel total-internal-reflection-fluorescence microscopy with a quartz-crystal microbalance (TIRFM-QCM) system.
The system has enabled researchers to see protein reactions on a quartz-crystal and protein movements in an evanescent field at the molecular level.
We group set a colorless and transparent quartz with high light permeability in protein solution and measured the vibrations of the quartz resonator with a pair of line antennas located above the flow channel, which generated and indirectly detected (without contact) the pure-shear vibrations of the quartz resonator from the electromagnetic waves.+]
Using this system, we monitor the deposition reaction of Aβ peptides on immobilized seeds grown from Aβ, which caused formation of oligomers in the early stage, and fibrillation procedure. This system allows simultaneous evaluation of the amount of deposited peptides on the surface seeds by QCM and fibril nucleation and elongation by TIRFM.
We also has found that a fibril-elongation rate two-orders-of-magnitude higher in an oligomeric cloud than reported values, indicating ultrafast transition of oligomers into fibrils. Our results will make a great contribution to drug development and correct diagnosis.
Interaction between monomer peptides and seeds is essential for clarifying the fibrillation mechanism of amyloid b (Ab) peptides. We monitored the deposition reaction of Ab1–40 peptides on immobilized seeds grown from Ab1–42, which caused formation of oligomers in the early stage. The deposition reaction and fibrillation procedure were monitored throughout by novel total-internal-reflection-fluorescence microscopy with a quartz-crystal microbalance (TIRFM-QCM) system. This system allows simultaneous evaluation of the amount of deposited peptides on the surface seeds by QCM and fibril nucleation and elongation by TIRFM. Most fibrils reached other nuclei, forming the fibril network across the nucleus hubs in a short time. We found a fibril-elongation rate two-orders-of-magnitude higher in an oligomeric cloud than reported values, indicating ultrafast transition of oligomers into fibrils.
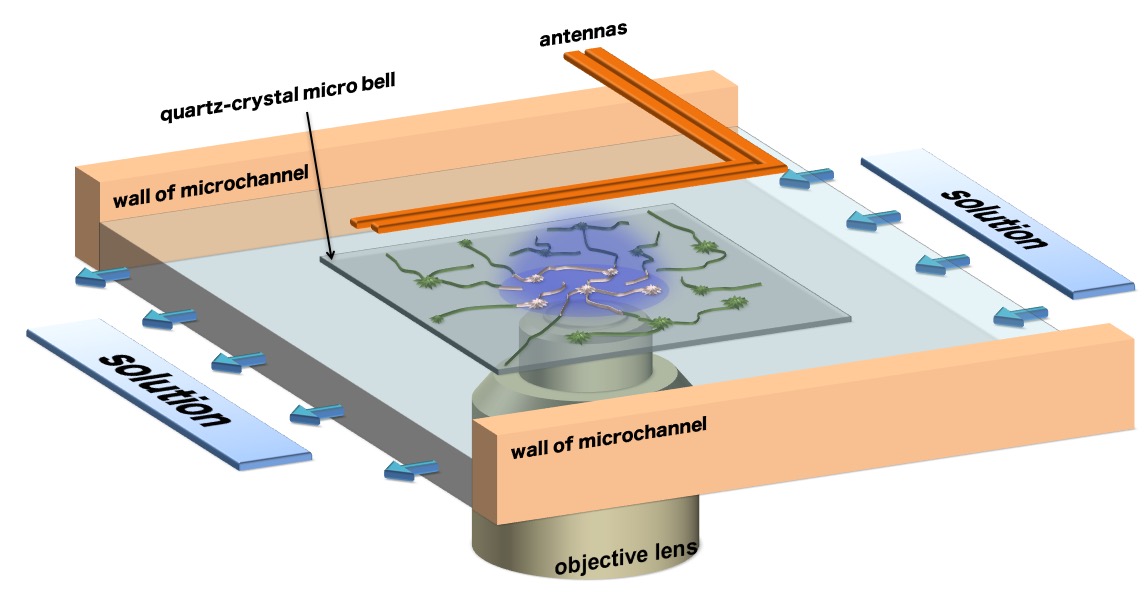
Figure 1 To learn more about this research, please view the full research report entitled "Ultrafast propagation of β-amyloid fibrils in oligomeric cloud" at of Scientific Reports website.